From Bioblast
| News and Events | Working Groups | Short-Term Scientific Missions | Management Committee | Members |
COST Action CA15203 (2016-2021): MitoEAGLE
Evolution-Age-Gender-Lifestyle-Environment: mitochondrial fitness mapping
The protonmotive force and respiratory control
File:OXPHOS system.jpg
Fig. 1. The mitochondrial respiratory system. In oxidative phosphorylation the electron transfer system (A) is coupled to the phosphorylation system (B). See Eqs. 4 and 5 for further explanation.
Mitochondrial respiratory control: a conceptual perspective on coupling states in mitochondrial preparations
- MitoEAGLE recommendations Part 1.
- » Work in progress: Download last update (Version 21') 2017-08-22:
 - » Versions
- » Versions
- » Work in progress: Download last update (Version 21') 2017-08-22:
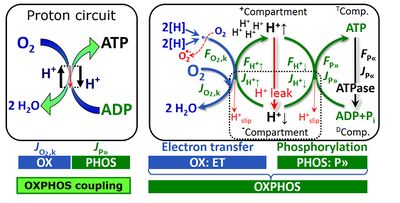
Fig. 2. The proton circuit and coupling in oxidative phosphorylation (OXPHOS). Modified after Gnaiger 2014 MitoPathways.
- MitoEAGLE Terminology Group
- First draft, updates, and corresponding author: E. Gnaiger
- Contributing co-authors: Confirming to have read the final manuscript, possibly to have made additions or suggestions for improvement, and to agree to implement the recommendations into future manuscripts, presentations and teaching materials. (alphabetical, to be extended):
- M.G. Alves, D. Ben-Shachar, G.C. Brown, G.R. Buettner, E. Calabria, Z. Cervincova, A.J. Chicco, P.M. Coen, J.L. Collins, L. Crisóstomo, M.S. Davis, C. Doerrier, E. Elmer, A. Filipovska, P.M. Garcia-Roves, B.H. Goodpaster, D.K. Harrison, K.T. Hellgren, C.L. Hoppel, J. Iglesias-Gonzalez, B.A. Irving, S. Iyer, P. Jansen-Dürr, T. Komlodi, V. Laner, H.K. Lee, H. Lemieux, A.T. Meszaros, N. Moisoi, A. Molina, A.L. Moore, A.J. Murray, J. Neuzil, R.K. Porter, K. Nozickova, P.J. Oliveira, K. Renner-Sattler, J. Rohlena, D. Salvadego, L.A. Sazanov, O. Sobotka, R. Stocker, I. Szabo, M. Tanaka, B. Tandler, L. Tretter, B. Velika, A.E. Vercesi, Y.H. Wei
- MitoEAGLE Terminology Group
- Supporting co-authors: Confirming to have read the final manuscript, and to agree to implement the recommendations into future manuscripts, presentations and teaching materials. (alphabetical, to be extended):
- P. Bernardi, R.A. Brown, T. Dias, G. Distefano, H. Dubouchaud, Z. Gan, L.F. Garcia-Souza, T. Käämbre, G. Keppner, A. Krajcova, M. Markova, J. Muntané, S Newsom, D. O'Gorman, M.T. Oliveira, C.M. Palmeira, D. Pesta, P.X. Petit, MM Robinson, K. Siewiera, P. Stankova, Z. Sumbalova, A. Zorzano
- Journal: Final decision has to be made; Int J Biochem Cell Biol, discussed at MITOEAGLE Barcelona 2017 and MiPschool Obergurgl 2017; page limit is surpassed; Open Access is a requirement.
New Table 3 in Version 21
- Contents
- 1. Introduction
- Mitochondrial preparations, mtprep,
- 1. Introduction
- 2. Fundamental respiratory coupling states in mitochondrial preparations
- 2.1. Definitions
- Phosphorylation, »P
- Control and regulation
- 2.2. Classical terminology for isolated mitochondria
- States 1-5
- 2.3. Three coupling states of mitochondrial preparations and residual oxygen consumption
- OXPHOS, ETS, LEAK, ROX
- 2.1. Definitions
- 3. States and rates
- 3.1. Respiratory states and respiratory rates
- 3.2. The steady-state and protonmotive force
- Protonmotive force, ∆pmt
- Faraday constant, F
- Electrical part of the protonmotive force, el
- Chemical diffusion (or dislocation) part of the protonmotive force, d
- Isomorph e [C]
- Isomorph n [mol]
- 3.3. Forces and flows in physics and irreversible thermodynamics
- Molar quantities
- Vectorial and scalar forces, and fluxes
- Coupled versus bound processes
- Coupling, efficiency, and power
- 3.4. Normalization: flows and fluxes
- Extensive quantities
- Size-specific quantities
- Flow per system, I
- Size-specific flux, J
- Flux per volume of the instrumental system, JV
- Sample concentration CmX and CNX
- Mitochondrial concentration, Cmt, and mitochondrial markers
- Mass-specific flux, JmX,O2
- Mitochondria-specific flux, Jmt,O2
- Flow per sample entity, IX,O2
- 3.5. Conversion: oxygen, protons, ATP
- 4. Conclusions
- Scope
- Target a broad audience – introduce the new generation of investigators.
- List of terms including historical terms; abbreviations (mtDNA, mt to abbreviate mitochondr*); OXPHOS capacity versus State 3 (discuss saturating ADP/Pi .. concentrations).
- Protonmotive force: not a force defined in physics, but an isomorphic force of statistical and nonequilibrium thermodynamics.
- Flux and flow: clarification of normalization.
- Scientific terminology should be general and platform-independent, meeting the demands of all working groups.
- Scope
Abstract
- Clarity of concepts and consistency of nomenclature are trademarks of the quality of a research field across its specializations, facilitating transdisciplinary communication and education. As research and knowledge on mitochondrial physiology expand, the necessity for harmonization of nomenclature on mitochondrial respiratory states and rates has become apparent. Peter Mitchell’s concept of the protonmotive force establishes the link between the electric and chemical components of energy transformation and coupling in oxidative phosphorylation. This unifying concept provides the framework for developing a consistent terminology on mitochondrial physiology and bioenergetics. We follow IUPAC guidelines on general terms of physical chemistry, extended by concepts of open systems and irreversible thermodynamics. The nomenclature of classical bioenergetics on respiratory states 1 to 5 in an experimental protocol is incorporated into a concept-driven constructive terminology to address the meaning of each respiratory state. Hence we focus primarily on the conceptual ‘why’ along with clarification of the experimental ‘how’. The capacity of oxidative phosphorylation, OXPHOS, provides diagnostic reference values and is, therefore, measured at kinetically saturating concentrations of ADP and inorganic phosphate. The contribution of intrinsically uncoupled oxygen consumption is most easily studied by arresting phosphorylation, when oxygen consumption compensates mainly for the proton leak, and the corresponding states are collectively classified as LEAK states. The oxidative capacity of the electron transfer system, ETS, reveals the limitation of OXPHOS capacity mediated by the phosphorylation system. Experimental standards for evaluation of respiratory coupling states must be followed for the development of databases of mitochondrial respiratory function.
- ‘Every professional group develops its own technical jargon for talking about matters of critical concern. .. People who know a word can share that idea with other members of their group, and a shared vocabulary is part of the glue that holds people together and allows them to create a shared culture’ (Miller 1991).
Classical terminology for isolated mitochondria
Three fundamental coupling states of mitochondrial preparations and residual oxygen consumption
States and rates
Normalization: fluxes and flows
List of selected terms and symbols
- Coupled respiration
- Dyscoupled respiration
- Isolated mitochondria, imt
- Mitochondria, mt (Greek mitos: thread; chondros: granule) are small structures within cells, which function in cell respiration as powerhouses or batteries. Mitochondria belong to the bioblasts of Richard Altmann (1894). Abbreviation: mt, as generally used in mtDNA. Singular: mitochondrion (bioblast); plural: mitochondria (bioblasts).
- Mitochondrial inner membrane, mt-im
- Mitochondrial outer membrane, mt-om
- Mitochondrial matrix, mt-matrix
- Mitochondrial membrane potential, mtMP, Δψmt
- Mitochondrial preparations, mtprep are isolated mitochondria (imt), tissue homogenate (thom), mechanically or chemically permeabilized tissue (permeabilized fibres, pfi) or permeabilized cells (pce). In these preparations the cell membranes are either removed (imt and smtp) or mechanically (thom) and chemically permeabilized (pfi), while the mitochondrial functional integrity and to a large extent the mt-structure is maintained.
- Mitochondrial respiration
- Noncoupled respiration
- Oligomycin, Omy
- Permeabilized tissue or cells, pti, pce
- Phosphorylation system
- Proton leak
- Proton pump
- Proton slip
- Protonmotive force, Δpmt
- Tissue homogenate, thom
- Uncoupler, U
Mitochondrial respiratory control: pathway states in mt-preparations
- MitoEAGLE recommendations Part 2.
- ‘It is essential to define both the substrate and ADP levels in order to identify the steady-state condition of the mitochondria during the experiment’ (Chance and Williams, 1956).
- The mitochondrial respiratory system
- Substrates and inhibitors
- Switch to pathway-related nomenclature instead of enzyme-linked terminology (N/NS/S versus CI/CI+II/CII)
Mitochondrial respiratory control: cell respiration
- MitoEAGLE recommendations Part 3.
- Intact cells versus mitochondrial preparations
- Intact cells, ce
- Basal respiration
- Cell respiration
- Resting metabolic rate
- ROUTINE state, state R: ROUTINE respiration of intact, viable cells is regulated according to physiological activity, at intracellular non-saturating ADP levels. R increases under various conditions of activation. When incubated in culture medium, cells maintain a ROUTINE level of activity, R (ROUTINE mitochondrial respiration; corrected for residual oxygen consumption due to oxidative side reactions). ROUTINE activity may include aerobic energy requirements for cell growth and is thus fundamentally different from the definition of basal metabolic rate (BMR). When incubated for short experimental periods in a medium devoid of fuel substrates, the cells respire solely on endogenous substrates at the corresponding state of ROUTINE activity, eR (e, endogenous substrate supply).
- Intact cells versus mitochondrial preparations
- It is difficult to stimulate living cells to maximum OXPHOS activity, since ADP and inorganic phosphate do not equilibrate across intact plasma membranes, and thus saturating concentrations of these metabolites can hardly be achieved in living cells. LEAK and ETS states, however, can be induced in viable cells with application of inhibitors of the phosphorylation system and uncouplers, respectively, due to the fact that cell membranes are highy permeable for these substances. External fuel substrates are taken up by living cells to various extents, and intracellular metabolism of exogenous and endogenous substrates supports mitochondrial respiration with a physiological substrate supply. In contrast, mt-preparations depend on the external supply of fuel substrates which support the electron transfer system with reducing equivalents. ETS competence of external substrates is required for all coupling states of mt-preparations (L, P, E) and depends on (i) transport of substrates across the inner mt-membrane or oxidation by dehydrogenases located on the outer face of the inner mt-membrane (e.g. glycerophosphate dehydrogenase complex, CGpDH), (ii) oxidation in the mt-matrix (TCA cycle dehydrogenases and other matrix dehydrogenases, e.g. mtGDH) or on the inner face of the inner mt-membrane (succinate dehydrogenase, CII), (iii) oxidation of substrates without accumulation of inhibitory endproducts (e.g. oxaloacetate inhibiting succinate dehydrogenase; NADH and oxaloacetate inhibiting malate dehydrogenase), and (iv) electron transfer through the membrane-bound ETS (mETS). Endproducts must be either easily exported from the matrix across the inner mt-membrane (e.g. malate formed from succinate via fumarate), or metabolized in the TCA cycle (e.g. malate-derived oxaloacetate forming citrate in the presence of external pyruvate&malate).
Mitochondrial respiratory control: coupling control ratios and control factors
- MitoEAGLE recommendations Part 4.
- ETS coupling efficiency, j≈E: j≈E = ≈E/E = (E-L)/E = 1-L/E
- LEAK control ratio, L/E
- Excess E-P capacity factor, jExP: jExp = (E-P)/E = 1-P/E
- OXPHOS control ratio, P/E
- OXPHOS coupling efficiency, j≈P: j≈P = ≈P/P = (P-L)/P = 1-L/P
- L/P coupling control ratio, L/P
- Respiratory acceptor control ratio, RCR: RCR = State 4/State 3
- netOXPHOS control ratio, ≈P/E: ≈P/E = (P-L)/E
- Uncoupling control ratio, UCR: UCR = E/R
Action
- » WG1 Action - WG1 MITOEAGLE protocols, terminology, documentation: Standard operating procedures and user requirement document: Protocols, terminology, documentation
- » WG1 Project application
- » 2017-07 MiPschool Obergurgl 2017
- » 2017-03 MITOEAGLE Barcelona 2017
- » 2016-11 MITOEAGLE 2016 Verona IT





