Rosca 2012 Diabetes
| Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL (2012) Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 61:2074-83. https://doi.org/10.2337/db11-1437 |
Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL (2012) Diabetes
Abstract: Mitochondrial reactive oxygen species (ROS) cause kidney damage in diabetes. We investigated the source and site of ROS production by kidney cortical tubule mitochondria in streptozotocin-induced type 1 diabetes in rats. In diabetic mitochondria, the increased amounts and activities of selective fatty acid oxidation enzymes is associated with increased oxidative phosphorylation and net ROS production with fatty acid substrates (by 40% and 30%, respectively), whereas pyruvate oxidation is decreased and pyruvate-supported ROS production is unchanged. Oxidation of substrates that donate electrons at specific sites in the electron transport chain (ETC) is unchanged. The increased maximal production of ROS with fatty acid oxidation is not affected by limiting the electron flow from complex I into complex III. The maximal capacity of the ubiquinol oxidation site in complex III in generating ROS does not differ between the control and diabetic mitochondria. In conclusion, the mitochondrial ETC is neither the target nor the site of ROS production in kidney tubule mitochondria in short-term diabetes. Mitochondrial fatty acid oxidation is the source of the increased net ROS production, and the site of electron leakage is located proximal to coenzyme Q at the electron transfer flavoprotein that shuttles electrons from acyl-CoA dehydrogenases to coenzyme Q.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
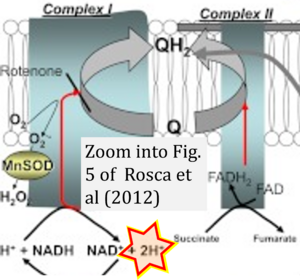
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
- Fig. 5 of Rosca et al (2012): Oxidation of succinate to fumarate reduces ubiquinone to ubiquinol through oxidation of FAD to FADH2 and further redox steps in CII. Likewise, oxidation of NADH + H+ to NAD+ reduces ubiquinone to ubiquinol through oxidation of FMN to FMNH2 and further redox steps in CI. This reduction of UQ to UQH2 effectively consumes the 2H+ together with 2e-. Hence, the 2H+ should not be shown to be formed in the matrix. Indication of H+-linked electron transfer as 2{H++e-} eliminates the ambiguity.
Labels: MiParea: Respiration Pathology: Diabetes Stress:Oxidative stress;RONS
Pathway: F