Knottnerus 2018 Rev Endocr Metab Disord
| Knottnerus SJG, Bleeker JC, Wüst RCI, Ferdinandusse S, IJlst L, Wijburg FA, Wanders RJA, Visser G, Houtkooper RH (2018) Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev Endocr Metab Disord 19:93-106. https://doi.org/10.1007/s11154-018-9448-1 |
Knottnerus SJG, Bleeker JC, Wuest RCI, Ferdinandusse S, IJlst L, Wijburg FA, Wanders RJA, Visser G, Houtkooper RH (2018) Rev Endocr Metab Disord
Abstract: Mitochondrial fatty acid oxidation is an essential pathway for energy production, especially during prolonged fasting and sub-maximal exercise. Long-chain fatty acids are the most abundant fatty acids in the human diet and in body stores, and more than 15 enzymes are involved in long-chain fatty acid oxidation. Pathogenic mutations in genes encoding these enzymes result in a long-chain fatty acid oxidation disorder in which the energy homeostasis is compromised and long-chain acylcarnitines accumulate. Symptoms arise or exacerbate during catabolic situations, such as fasting, illness and (endurance) exercise. The clinical spectrum is very heterogeneous, ranging from hypoketotic hypoglycemia, liver dysfunction, rhabdomyolysis, cardiomyopathy and early demise. With the introduction of several of the long-chain fatty acid oxidation disorders (lcFAOD) in newborn screening panels, also asymptomatic individuals with a lcFAOD are identified. However, despite early diagnosis and dietary therapy, a significant number of patients still develop symptoms emphasizing the need for individualized treatment strategies. This review aims to function as a comprehensive reference for clinical and laboratory findings for clinicians who are confronted with pediatric and adult patients with a possible diagnosis of a lcFAOD.
• Bioblast editor: Gnaiger E
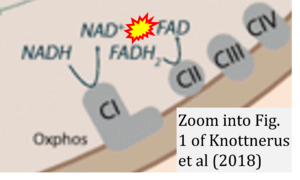
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase
Pathway: F