Description
An elementary entity UX [x] of entity type X is a single unit of countable objects (X = molecules, cells, organisms, particles, parties) or events (X = beats, collisions, emissions, decays, celestial cycles, instances, occurences, parties). "An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles" (Bureau International des Poids et Mesures 2019).
If an object is defined as an assembly of particles (a party of two, a molecule as the assembly of a stoichiometric number of atoms), then the entity is the assembly but not the assembled particle. A number of defined elementary units UX is a count, NX = N·UX [x], where N is only a number and as such N is dimensionless. The elementary unit UX has the dimension U of the count NX. The elementary unit UX has the same unit [x] as the count NX, or more accurately it gives the count the defining 'counting-unit' [x]. From the definition of count as the number (N) of elementary units (U) of entity type X, it follows that count divided by elementary unit is simply a number, N = NX·UX-1. The unit uN of a count can neither be the entity X nor a number. The elementary entity type X defines the identity X of the elementary unit UX with the unit 'counting-unit' with symbol [x]. Since a count NX is the number of elementary entities, the elementary entity UX is not a count (UX; it is not identical with N·UX).
Abbreviation: UX [x]
Reference: BEC2020.1 doi10.26124bec2020-0001.v1
Communicated by Gnaiger Erich 2020-07-10 in: Anastrophe XX Entity X and the elementary unit x of A X-mass Carol
Entity-type, elementary entity, and elementary unit
- In Euclid's Elements (Book VII) a "unit" is defined as 'a single individual thing'. Take 'individual thing' as equal to elementary entity. The Euclidean unit is tightly conjugated with the individual thing. In diametrical contrast, the SI unit is entirely abstracted from any individual type of thing. The SI unit is entirely disjugated from the individual thing. Put 1 SI unit into an experimental system to work with a volume of 1 unit [m3] or run an experiment with a mass of 1 unit [kg], it may be a unit of anything. 1 kg is always 1 kg of any entity X. The entity-type X does not even have to be known to measure volume in units of meters cubed or mass in units of kg.
- We see Euclid's unit in reflections with the SI's unit not merely in a looking glass, but in a kaleidoscopic interplay of Number, Unit, Count, Entity — NUCE (in nuce), which remains a CASE to this day (CASE represents the Counting-Assembling-Sorting Experience).
- Entity-type X is distinguished from the elementary entity UNX. Dual-message code may lead to misunderstanding depending on context. For example, take O2 as entity X. VO2 and mO2 are the volume [m3] and mass [kg], respectively, of a sample of pure O2. For the volume, it is important to specify the gaseous state at a given temperature and pressure, whereas these specifications are irrelvant for the quantity mass. For volume and mass it needs to be ensured that there is a pure sample of oxygen (X = oxygen), but the specification of the elementary entity is irrelevant. The values of oxygen volume and oxygen mass remain independent of having in mind molecules of O2, atoms of O, or charges of oxygen. Observe now the subtle transition from extensive quantities Qu (VO2 and mO2) to elementary quantities QU (NO2 and nO2).
- NO2 is the count of O2 molecules. NO2 is equal to the number of elementary entities UO2. The elementary entity UO2 for the count of oxygen molecules is different from the elementary entity UO for the count of oxygen atoms. UO2 and NO2 are expressed in the unit 'elementary unit' [x]. nO2 is the amount of O2 expressed in the unit mole [mol]. nO2 is equal to NA-1 times the number of elementary entities UO2.
- X = ce in 'cell mass' is normally understood as meaning "the mass of all entities of entity-type ce in a sample", mce [kg]. In contrast, X = body in the context of body mass and body mass index (BMI) is used in dual-message code, mbody. Body mass with dual-message code means the mass of all entities of entity-type human body, mbody [kg], divided by the number of bodies, Nbody, which leads to the explicit canonical expression MUbody = mbody·Nbody-1.
- Colour code: Colour red may be used in X, indicating that the term entity or symbol X are used in dual-message code, meaning both entity-type and unit-entity. When specifying "single entity X" it is sufficiently clear that X is used in dual-message mode, and the term "number of X" is equally clear to meaning "number of unit-entities X", NX = N·UNX-1. Similarly, in the term "O2 flow per cell" IO2/ce" it is sufficiently clear that ce is used in in dual-message code.
Base quantities and count
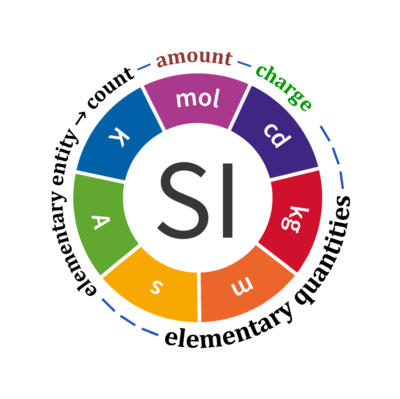
Quantity Symbol for quantity Q Symbol for dimension Name of abstract unit uQ Symbol for unit uQ [*] elementary entity *,$ UX U elementary unit x count *,$ NX = N·UX X elementary unit x amount of substance *,§ nX = NX·NA-1 N mole mol charge *,€ Qel = zX·e·NX I·T coulomb C = A·s length l L meter m mass m M kilogram kg time t T second s electric current I I ampere A thermodynamic temperature T Θ kelvin K luminous intensity Iv J candela cd
- [*] SI units, except for the canonical 'elementary unit' [x]. The following footnotes are canonical comments, related to iconic symbols.
- * For the elementary quantities NX, nX, and Qel, the entity-type X of the elementary entity UX has to be specified in the text and indicated by a subscript: nO2; Nce; Qel.
- $ Count NX equals the number of elementary entities UX. In the SI, the quantity 'count' is explicitly considered as an exception: "Each of the seven base quantities used in the SI is regarded as having its own dimension. .. All other quantities, with the exception of counts, are derived quantities" (Bureau International des Poids et Mesures 2019 The International System of Units (SI)). An elementary entity UX is a material unit, it is not a count (UX is not a number of UX). NX has the dimension X of a count and UX has the dimension U of an elementary entity; both quantities have the same abstract unit, the 'elementary unit' [x].
- § Amount nX is an elementary quantity, converting the elementary unit [x] into the SI base unit mole [mol] using the Avogadro constant NA.
- € Charge is a derived SI quantity. Charge is an elementary quantity, converting the elementary unit [x] into coulombs [C] using the elementary charge e, or converting moles [mol] into coulombs [C] using the Faraday constant F. zX is the charge number per elementary entity UX, which is a constant for any defined elementary entity UX. Qel = zX·F·nX
References
| Bioblast link | Reference | Year |
|---|---|---|
| Gnaiger 2020 BEC MitoPathways | Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | 2020 |
| Gnaiger 2020 MitoFit x | Gnaiger E (2021) The elementary unit — canonical reviewer's comments on: Bureau International des Poids et Mesures (2019) The International System of Units (SI) 9th ed. https://doi.org/10.26124/mitofit:200004.v2 | 2021 |
| BEC 2020.1 doi10.26124bec2020-0001.v1 | Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | 2020 |
- Bioblast links: SI base units - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Entity, count, and number, and SI base quantities / SI base units
Quantity name Symbol Unit name Symbol Comment elementary UX elementary unit [x] UX, UB; [x] not in SI count NX elementary unit [x] NX, NB; [x] not in SI number N - dimensionless = NX·UX-1 amount of substance nB mole [mol] nX, nB electric current I ampere [A] A = C·s-1 time t second [s] length l meter [m] SI: metre mass m kilogram [kg] thermodynamic temperature T kelvin [K] luminous intensity IV candela [cd]
- Fundamental relationships
- » Avogadro constant NA
- » Boltzmann constant k
- » elementary charge e
- » Faraday constant F
- » gas constant R
- » electrochemical constant f
- Fundamental relationships
- SI and related concepts
MitoPedia concepts:
Ergodynamics