Carboxy SNARF 1: Difference between revisions
From Bioblast
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|info=https://www.thermofisher.com/order/catalog/product/S22801 | |info=https://www.thermofisher.com/order/catalog/product/S22801 | ||
}} | }} | ||
:::: Life technologies (Invitrogen): "Stock solutions can be made in water or buffer and should be divided into aliquots and stored frozen at <=-20 °C, protected form light." | :::: Life technologies (Invitrogen): "Stock solutions can be made in water or buffer and should be divided into aliquots and stored frozen at <=-20 °C, protected form light." | ||
| Line 61: | Line 60: | ||
::::* Medium: Hank's mod.3 | ::::* Medium: Hank's mod.3 | ||
::::* Cell density 1/2/3/4 Mill. cells/mL | ::::* Cell density 1/2/3/4 Mill. cells/mL | ||
{{Keywords: pH}} | |||
{{MitoPedia methods | {{MitoPedia methods | ||
|mitopedia method=Fluorometry | |mitopedia method=Fluorometry | ||
}} | }} | ||
Latest revision as of 01:03, 18 February 2020
Description
Carboxy SNARF® 1 is a cell-impermeant pH indicator dye. The pKa of ~7.5 makes it useful for measuring pH in the range of pH 7 to pH 8. The emission shifts from yellow-orange at low pH to deep red fluorescence at high pH. Ratiometric fluorometry, therefore, is applied at two emission wavelengths,such as 580 nm and 640 nm.
Relative molecular mass: Mr = 453.45
Abbreviation: SNARF
Reference: https://www.thermofisher.com/order/catalog/product/S22801
- Life technologies (Invitrogen): "Stock solutions can be made in water or buffer and should be divided into aliquots and stored frozen at <=-20 °C, protected form light."
- Solution used by the producer for specific measurements: 50 mM potassium phosphate, pH 10.
- Solution used for measurement in patent of producer: 40 mM potassium phosphate, pH 9, probable just K2HPO4 solution.
- Since solubility in water at neutral pH is not known, a high pH>=9 solution should be used to ensure a proper solution state.
Carboxy SNARF 1 with the NextGen-O2k
Stock solution
- Lot Nr. 1189785 (2014-07-08)
- 1 mg in 1.1 mL 50 mM K2HPO4 solution (pH at RT ca 9.3) --> 2 mM
- 100 µL aliquots in 0.5 mL Eppendorf, store at -20 °C.
Calibration
- Calibration buffers
- >= 3 calibration points of known pH value are required.
- Basic endpoint: fluorophore completely deprotonated.
- Acidic endpoint: fluorophore completly protonated.
- The concept of calibration buffers was evaluated critically, because
- using different media for calibration ignores the changes in optical properties due to the sample;
- small differences in pH value are necessary that ideally should be measured in the chamber anyway.
- The concept of calibration buffers was evaluated critically, because
- Injection of acid / base during the experiment
- Inject small amounts of acid or base into the chamber and measure the resulting pH directly in the chamber with a potentiometric pH electrode. Repeated opening and closing of the chamber is necessary, since the pH electrode has to be removed during fluorometric measurement.
- For the calculations the fluorescence signal at the complete protonated and deprotonated forms of the fluorophore are required (endpoints). These states are established experimentally at the end of the experiment by injecting strong acids and bases. To avoid precipitation (and thereby changing optical properties) the following points have to be considered:
- Coagulation of cells in alkaline solution: see
- Precipitation of Mg(OH)2 and Ca(OH): Mg(OH)2 has the a lower solubility and is therefore the limiting factor of these two: KL Mg(OH)2 at ca 20° 10-11. c(Mg) in e.g. Hanks mod buffer ca 0.5 mM. Precipitations should start only above pH > 10. The limiting factor would thereby be the coagulation of cells (but also search KL for higher Temp).
- Precipitation of fluorophore at acidic conditions: the fluorophore precipitates when completely protonated. The acidic endpoint should therefore be measured last and immediately after injecting the acid.
Hanks based calibration buffers
Toxicity SNARF
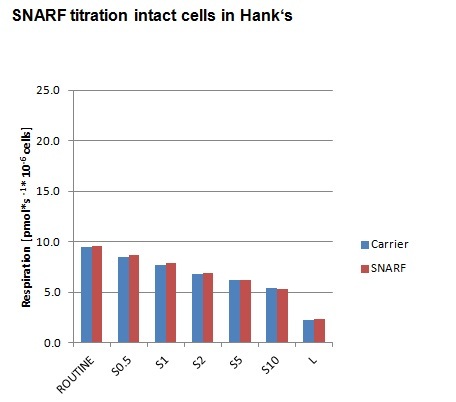
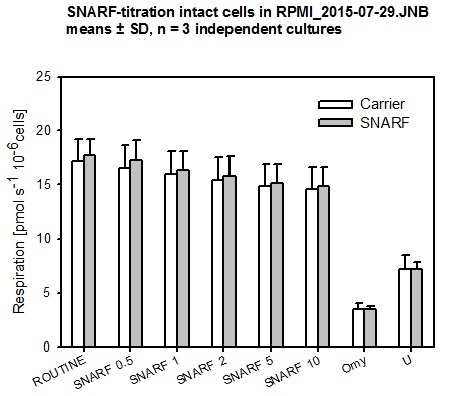
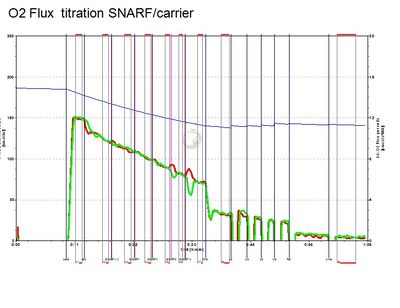
- At final SNARF concetrations of 0.5 / 1 / 2 / 5 / 10 µM, no inhibition of respiration of HEK 293T cells was observed.
- Experimental code MA 154a
- O2k Settings: green LED; light intensity = 4 mA; data sampling interval: 2 s; integration time: 100; detection wavelength range 1: 580-600 nm, range 2: 620–685 nm.
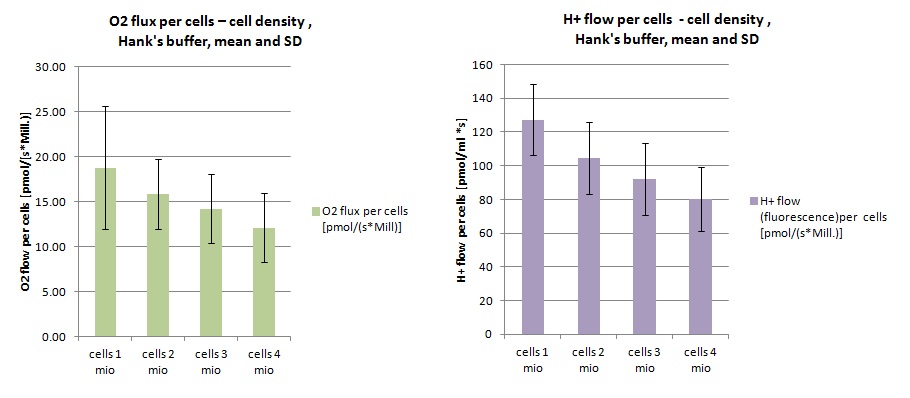
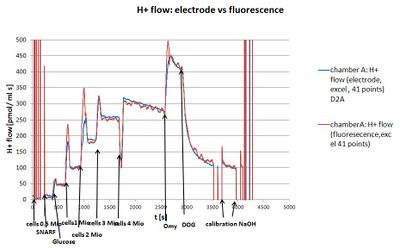
Optimum cell density
- At a cell density of 3 Mill cells/mL a good step change in the fluorescence signal was observed. Thus further experiments were carried out with this cell density.
- Experimental codes: MA 155 PD2/PD5, MA157 PD2/PD5, MA 160 PD5
- HEK 293 T cells:
- Medium: Hank's mod.3
- Cell density 1/2/3/4 Mill. cells/mL
- Bioblast links: pH and protons - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- pH and protons
- » pH
- » hydrogen ion H+
- » hydron H+
- » hydronium ion H3O+
- » hydride H-
- » proton p+
- » pH buffering capacity
- » proton flux
- » proton pump versus hydrogen ion pump
- » proton leak
- » proton slip
- » protonmotive force
- pH and protons
- O2k-pH
- » O2k-Catalogue: O2k-pH ISE-Module
- » O2k-Manual pH electrode: MiPNet23.15 O2k-pH ISE-Module
- » O2k-SOP: MiPNet08.16 pH calibration
- » File:PH-Calibration-List.xls
- » NextGen-O2k, ratiometric: Carboxy SNARF 1
- » NextGen-O2k, ratiometric: HPTS
- » pH calibration buffers
- O2k-pH
- O2k-Publications
- HRFR - general
- » O2k-Manual: MiPNet22.11 O2k-FluoRespirometer manual
- » O2k signals and output
- » O2k-SOP: MiPNet14.06 Instrumental O2 background
- » MiPNet19.18A O2k-Series G: Start
- » ESD
- » O2k configuration
- » O2k control
- » O2k-FluoRespirometer
- » O2k-Main Unit#O2k-Series
- » Titration-Injection microPump
- » Compare: O2k-TPP+_ISE-Module
- HRFR - general
- DatLab
MitoPedia methods:
Fluorometry