Schon 2018 Science
| Schon EA (2018) Bioenergetics through thick and thin. Science 362:1114-5. https://doi.org/10.1126/science.aav7629 |
Abstract: The cells in all biological systems are composed of a limited number of molecular constituents, mainly proteins, nucleic acids, carbohydrates, and lipids. Of these, lipids tend to receive the shortest shrift, as they are typically considered to be merely the building blocks of membranes that provide a scaffold in which the “important” molecules, such as enzymes or signaling proteins, reside. In recent years, and with the advent of advanced lipidomics techniques, we have learned that “a lipid is a lipid is a lipid” is simply not true, and that the lipid composition of membranes can have profound effects on the behavior and activity of its resident macromolecules. For example, some lipids, such as members of the phosphatidylinositol family, are important signaling molecules. However, what is less appreciated is that the physical composition of lipid membranes can have profound effects on cellular behavior as well. On page 1186 of this issue, Budin et al. (1) use the Gram-negative bacterium Escherichia coli and the budding yeast Saccharomyces cerevisiae as model systems to show that the fluidity of a membrane, as determined by its lipid composition, can have huge effects on the efficiency of aerobic energy production (respiration) by the highly hydrophobic, membrane-embedded, oxidative phosphorylation (OxPhos) system.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
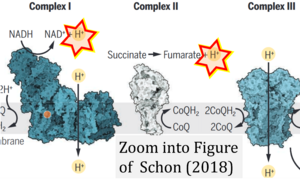
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.