Rai 2022 G3 (Bethesda)
| Rai M, Carter SM, Shefali SA, Mahmoudzadeh NH, Pepin R, Tennessen JM (2022) The Drosophila melanogaster enzyme glycerol-3-phosphate dehydrogenase 1 is required for oogenesis, embryonic development, and amino acid homeostasis. G3 (Bethesda) 12:jkac115. https://doi.org/10.1093/g3journal/jkac115 |
Rai M, Carter SM, Shefali SA, Mahmoudzadeh NH, Pepin R, Tennessen JM (2022) G3 (Bethesda)
Abstract: As the fruit fly, Drosophila melanogaster, progresses from one life stage to the next, many of the enzymes that compose intermediary metabolism undergo substantial changes in both expression and activity. These predictable shifts in metabolic flux allow the fly meet stage-specific requirements for energy production and biosynthesis. In this regard, the enzyme glycerol-3-phosphate dehydrogenase 1 (GPDH1) has been the focus of biochemical genetics studies for several decades and, as a result, is one of the most well-characterized Drosophila enzymes. Among the findings of these earlier studies is that GPDH1 acts throughout the fly lifecycle to promote mitochondrial energy production and triglyceride accumulation while also serving a key role in maintaining redox balance. Here, we expand upon the known roles of GPDH1 during fly development by examining how depletion of both the maternal and zygotic pools of this enzyme influences development, metabolism, and viability. Our findings not only confirm previous observations that Gpdh1 mutants exhibit defects in larval development, lifespan, and fat storage but also reveal that GPDH1 serves essential roles in oogenesis and embryogenesis. Moreover, metabolomics analysis reveals that a Gpdh1 mutant stock maintained in a homozygous state exhibits larval metabolic defects that significantly differ from those observed in the F1 mutant generation. Overall, our findings highlight unappreciated roles for GPDH1 in early development and uncover previously undescribed metabolic adaptations that could allow flies to survive the loss of this key enzyme.
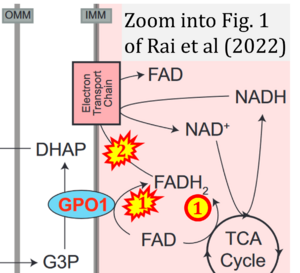
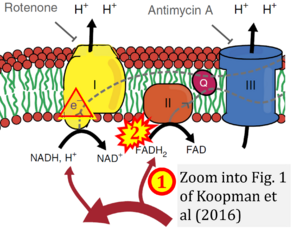
- Comment (Cardoso Luiza, Gnaiger Erich, 2023-08-05): Figure 1 shows FADH2 to be formed in the mitochondrial matrix from (1) GPO1 (CGpDH) and the TCA cycle, feeding electrons further (2) into the 'Electron Transport Chain' (ETS). Combined with FADH2 formed from (1) the TCA cycle and feeding (2) into CII (Fig. 1 on the right; one of >120 examples discussed as CII-ambiguities), one may arrive at the erroneous conclusion on a direct role of CII in the oxidation of glycerol-3-phosphate, analogous to false representations of CII involved in fatty acid oxidation.
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: MiParea: Developmental biology
Organism: Drosophila
Enzyme: Complex II;succinate dehydrogenase
Pathway: Gp