Koene 2011 J Inherit Metab Dis
| Koene S, Willems PH, Roestenberg P, Koopman WJ, Smeitink JA (2011) Mouse models for nuclear DNA-encoded mitochondrial complex I deficiency. J Inherit Metab Dis 34:293-307. https://doi.org/10.1007/s10545-009-9005-x |
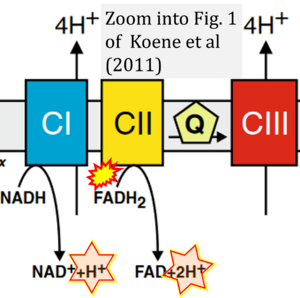
Koene S, Willems PH, Roestenberg P, Koopman WJ, Smeitink JA (2011) J Inherit Metab Dis
Abstract: Mitochondrial diseases are a group of heterogeneous pathologies with decreased cellular energy production as a common denominator. Defects in the oxidative phosphorylation (OXPHOS) system, the most frequent one in humans being isolated complex I deficiency (OMIM 252010), underlie this disturbed-energy generation. As biogenesis of OXPHOS complexes is under dual genetic control, with complex II being the sole exception, mutations in both nuclear DNA (nDNA) and mitochondrial DNA (mtDNA) are found. Increasing knowledge is becoming available with respect to the pathophysiology and cellular consequences of OXPHOS dysfunction. This aids the rational design of new treatment strategies. Recently, the first successful treatment trials were carried out in patient-derived cell lines. In these studies chemical compounds were used that target cellular aberrations induced by complex I dysfunction. Before the field of human clinical trials is entered, it is necessary to study the effects of these compounds with respect to toxicity, pharmacokinetics and therapeutic potential in suitable animal models. Here, we discuss two recent mouse models for nDNA-encoded complex I deficiency and their tissue-specific knock-outs.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.