Fromenty 2023 J Hepatol
| Fromenty B, Roden M (2023) Mitochondrial alterations in fatty liver diseases. J Hepatol 78:415-29. https://doi.org/10.1016/j.jhep.2022.09.020 |
Fromenty B, Roden M (2023) J Hepatol
Abstract: Fatty liver diseases can result from common metabolic diseases, as well as from xenobiotic exposure and excessive alcohol use, all of which have been shown to exert toxic effects on hepatic mitochondrial functionality and dynamics. Invasive or complex methodology limits large-scale investigations of mitochondria in human livers. Nevertheless, abnormal mitochondrial function, such as impaired fatty acid oxidation and oxidative phosphorylation, drives oxidative stress and has been identified as an important feature of human steatohepatitis. On the other hand, hepatic mitochondria can be flexible and adapt to the ambient metabolic condition to prevent triglyceride and lipotoxin accumulation in obesity. Experience from studies on xenobiotics has provided important insights into the regulation of hepatic mitochondria. Increasing awareness of the joint presence of metabolic disease-related (lipotoxic) and alcohol-related liver diseases further highlights the need to better understand their mutual interaction and potentiation in disease progression. Recent clinical studies have assessed the effects of diets or bariatric surgery on hepatic mitochondria, which are also evolving as an interesting therapeutic target in non-alcoholic fatty liver disease. This review summarises the current knowledge on hepatic mitochondria with a focus on fatty liver diseases linked to obesity, type 2 diabetes and xenobiotics.
• Bioblast editor: Gnaiger E
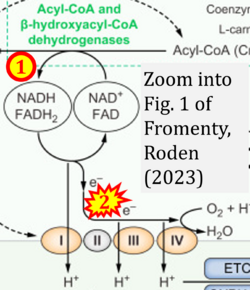
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase
Pathway: F